Liverpool firm develops breakthrough disease test
A Liverpool firm has filed a patent for a ‘game-changer’ novel technology that enables lateral flow tests, currently used for COVID-19 testing, to detect a range of other diseases. Tony McDonough reports

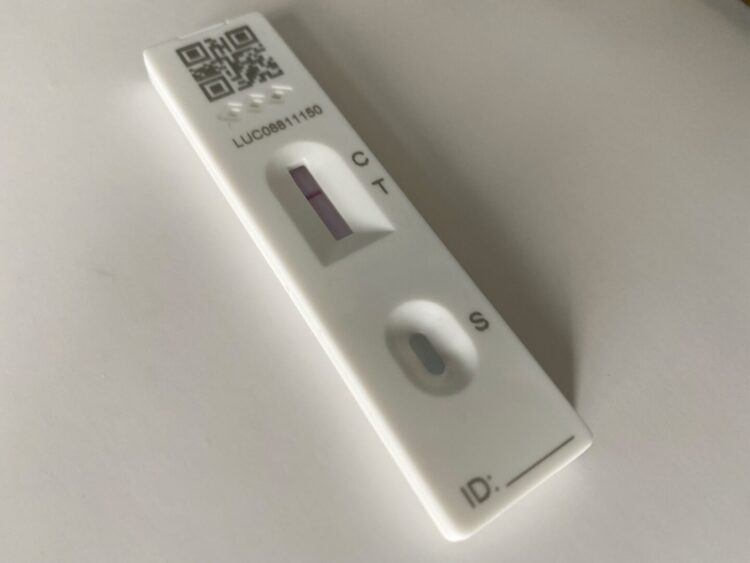
Lateral flow tests (LFTs) that are used by millions of people to test for COVID-19 have been adapted to test for a range of other diseases by a Liverpool firm.
Nano Biosols, based at Liverpool Science Park, has filed a patent for novel technology it has developed to enhance the sensitivity of LFTs – enabling their use across a much broader range of diseases.
Although lateral flow tests are a relatively cheap, user-friendly, and effective testing method, they are generally not as sensitive as the gold standard PCR test for detecting active infection.
Nano Biosols’ novel gold nanoparticle technology can improve the sensitivity and readability of lateral flow tests in cases where detection lines are only faintly displayed. This could be a game-change for the detection of a number of diseases.
The firm worked with the Liverpool city region support programme iiCON (Infection Innovation Consortium), and its partners at Liverpool School of Tropical Medicine (LSTM), to undertake initial testing and evaluation of its novel technology.
The diagnostics team at LSTM was able to evaluate lateral flow test performance with and without the addition of the Nano Biosols reagent and showed improved detection limits and readability of tests.
Adrian Walsh, director of Nano Biosols, said: “We’re very encouraged with the results of the tests that have been undertaken on our novel technology through the iiCON programme. These results have enabled the company to file for patent protection and we are looking forward to undertaking further evaluations.”
iiCON, which is led by LSTM, bridges the gap in the infection innovation ecosystem between industry, academia, and the NHS to accelerate and support the discovery and development of innovative new anti-infectives. It has so far raised £173m and created 176 jobs.
Dr Lisa Baldwin, iiCON’s senior business development manager, added: “Lateral flow tests are an incredibly important tool for diagnosing infection in communities and during the pandemic. People have become very familiar with them. However, these tests often don’t reach the gold standard of sensitivity that the more complex PCR tests offer.
“Nano Biosol’s novel technology has the potential to be a significant game-changer in infection diagnostics – enabling people to benefit from highly-sensitive, user-friendly tests that could be used to detect a huge range of infectious diseases.”
And Dr Ana Cubas Atienzar, post-doctoral research associate at LSTM, also said: ‘We’re very encouraged by the results of the tests we’ve conducted on Nano Biosols’ technology and the potential it has to significantly improve the performance of lateral flow tests.
“It could be added to any platform and be used for any target disease, so could represent a step-change in how we currently utilise lateral flow tests.”

