Daresbury firm secures US approval for lifesaving medical device
Based at Sci-Tech Daresbury, Sky Medical Technology will now look to roll out its ‘geko’ device to stroke patients across the Atlantic and tap into a $3.5bn market
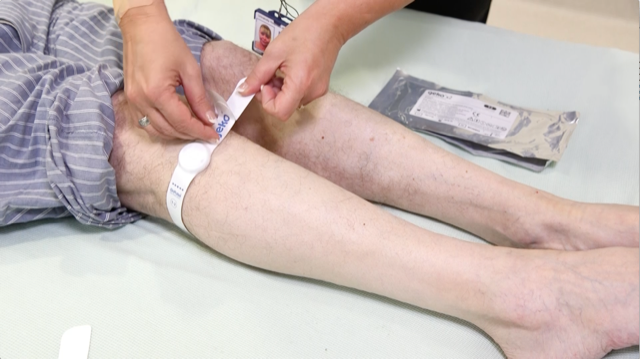
A Liverpool city region medical devices business has secured approval from US regulators to market its bioelectronic device that prevents life-threatening blood clots.
Based at Sci-Tech Daresbury, Sky Medical Technology will now look to roll out its ‘geko’ device to patients across the Atlantic and access a $3.5bn market for the prevention of blood clots in stroke patients.
The US Food and Drug Administration (FDA) has given the go-ahead for the use of the device to treat stroke patients. It had previously cleared the use of geko in increasing immediate post-surgical venous thromboembolism (VTE) prevention.
This latest approval makes the device the first bioelectronic muscle pump activator of its kind to be cleared by the FDA for VTE prevention across all patients, including non-surgical patients.
The geko is a non-invasive, easy to use, battery powered, wearable therapy device. The size of a wristwatch and worn at the knee, the daily disposable device gently stimulates the common peroneal nerve activating the calf and foot muscle pumps.
This results in increased blood flow in the deep veins of the calf. The geko device operates without external pressure to the leg and allows complete mobility.
Sky Medical Technology chief executive Bernard Ross said: “Our proven model of partnering with healthcare clinicians both at home and globally has been a key tenet to growing our export activity, and has ensured success in multiple markets and areas of clinical practice.
“This builds on our previous FDA indications to address life threatening blood clot conditions, and we are excited to extend our access in to the US market. This rapid growth is a result of collaboration with early adopters in the UK, which has generated crucial proven clinical and health economic data which is now translating internationally.
“New care pathways are in development and we plan to submit further FDA applications to expand our claims. We anticipate further international growth in the coming year.”
VTE is a deadly risk to hospitalised patients, particularly those who are immobile as a result of acute stroke or trauma recovery. Estimates suggest that 60,000 – 100,000 Americans die each year as a result of the condition. In England the estimate is 40,000 deaths annually.
